Introduction
Fenbendazole is a medication used to treat hookworms, roundworms, tapeworms, and other intestinal parasites in dogs. It belongs to the class of drugs known as benzimidazoles, which work by preventing parasitic worms from absorbing nutrients, essentially starving them. Fenbendazole was initially approved by the FDA for use in dogs in 1974.
For decades, veterinarians have prescribed fenbendazole as a safe and effective dewormer for dogs. It is available under brand names such as Panacur and Safe-Guard, often in chewable tablet form. At the standard veterinary dose for dogs, fenbendazole has a wide safety margin and minimal side effects.
While fenbendazole has become a widely used anthelmintic drug in veterinary medicine, its use in humans has been limited. But recent scientific research and anecdotal reports have spurred interest in repurposing this veterinary dewormer as an experimental cancer treatment for humans.
The Off-Label Use of Fenbendazole for Cancer
Fenbendazole is a drug commonly used as a deworming medication for dogs. Interestingly, some people have experimented with using fenbendazole off-label as a cancer treatment in humans. This unusual application of a veterinary medication stems from limited research indicating fenbendazole may have anti-cancer properties.

The notion of using fenbendazole to treat cancer originated within the Joe Tippens protocol. This experimental regimen gained attention after a cancer patient, Joe Tippens, claimed fenbendazole helped eliminate his small cell lung cancer. Tippens said he took a dog dewormer containing fenbendazole along with a few other supplements while declining conventional cancer treatments.
Tippens’ extraordinary story led many other cancer patients to follow his protocol, taking high doses of fenbendazole in addition to vitamins and other natural products. However, it’s important to note much of the enthusiasm for fenbendazole as an off-label cancer therapy is based on anecdotal reports. Rigorous scientific research on fenbendazole’s efficacy and safety for cancer is still quite limited.
The Proposed Mechanisms Behind Fenbendazole’s Anti-Cancer Effects
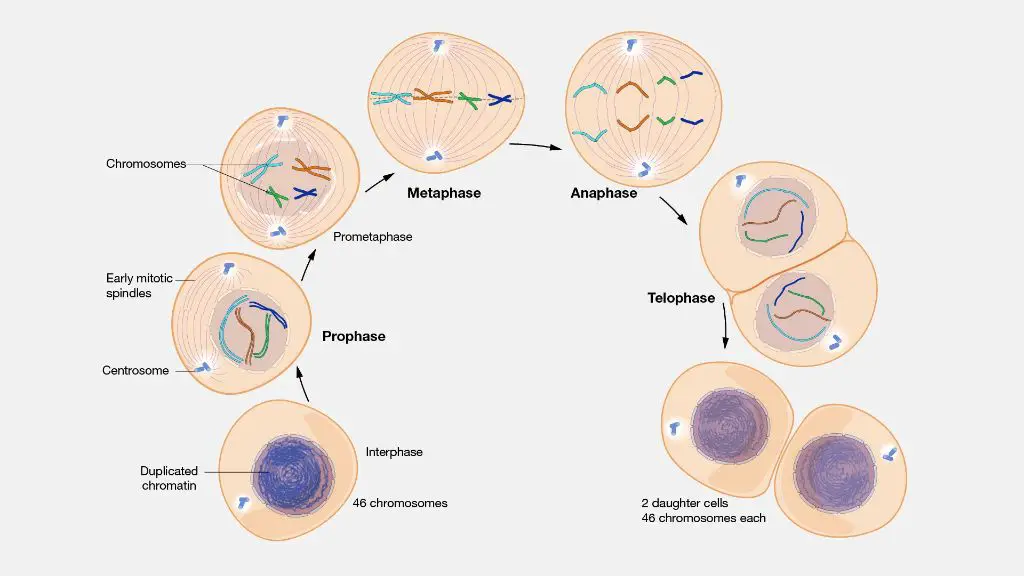
There are several hypotheses for how fenbendazole may inhibit tumor growth and angiogenesis:
- Fenbendazole may disrupt microtubule formation – Microtubules are important structural components of cells that are involved in cell division. Disrupting microtubule formation can inhibit cancer cell proliferation.
- It may inhibit glucose uptake – Cancer cells rely on increased glucose uptake and glycolysis for energy production. Fenbendazole may interfere with this process, essentially starving cancer cells.
- It could have anti-angiogenic effects – Angiogenesis, the growth of new blood vessels, is important for tumor growth and metastasis. Fenbendazole may block angiogenesis, restricting the blood supply to tumors.
- There is some evidence it causes apoptosis – Apoptosis is programmed cell death, and triggering apoptosis in cancer cells leads to tumor cell death. Fenbendazole may activate apoptotic pathways in cancer cells.
- It may modulate the immune system – Fenbendazole may enhance immune system activity against cancer cells. It may activate cytotoxic T cells or increase production of cytokines that inhibit tumor growth.
While the exact mechanisms are still being elucidated, fenbendazole appears to target multiple pathways important for cancer cell survival and tumor progression. Further research is needed to clarify the primary ways in which fenbendazole exerts anti-cancer effects.
Early Research Supporting the Anti-Cancer Effects of Fenbendazole
In the early 2000s, several in vitro and animal studies demonstrated the potential anti-cancer effects of fenbendazole. These studies laid the groundwork for further research into the off-label use of this common anti-parasitic drug for cancer treatment.
In 2002, researchers found that fenbendazole exhibited cytotoxic effects against cancer cell lines in vitro. The study showed that fenbendazole inhibited proliferation and induced apoptosis in human cancer cells of the lymphoblastoid, myeloid and prostate lines.
Another study published in 2003 revealed that fenbendazole displayed anti-tumor activity in vitro and inhibited tumor growth and metastases in mice inoculated with cancer cells. The drug was able to suppress tumor growth by more than 90% compared to control animals.
In 2006, a study reported that fenbendazole inhibited tumor cell viability, disrupted microtubule formation and induced apoptosis in human cancer cells lines. The researchers concluded that fenbendazole may be an effective anti-cancer drug through its perturbing effects on microtubule function.
Further preclinical studies in later years built upon these early findings and continued to demonstrate the potential utility of fenbendazole as a novel anti-cancer agent.
Anecdotal Reports of Fenbendazole’s Anti-Cancer Effects
There are many anecdotal patient testimonials and case reports describing the anti-cancer effects of fenbendazole. These reports come from cancer patients who decided to try fenbendazole off-label in a last ditch effort to treat their disease. Some of the cancers that patients report successfully treating with fenbendazole include glioblastoma, lymphoma, multiple myeloma, prostate cancer, lung cancer, and pancreatic cancer.
Many patients report that within months of taking a fenbendazole regimen, their tumors shrank significantly or disappeared altogether. In some cases, patients who were given just weeks or months to live have survived for years after starting on fenbendazole. They credit the drug with sending their cancer into remission when radiation, chemotherapy and other treatments failed.
These anecdotal reports have sparked interest in fenbendazole as an anti-cancer treatment. However, more rigorous clinical trials are needed to truly determine its efficacy and safety. The stories of cancer remission are encouraging, but may be attributable to other factors besides the fenbendazole treatment. Still, the remarkable responses reported by some patients merit further scientific investigation.
Ongoing Clinical Trials

While fenbendazole shows promise as an anti-cancer agent based on preclinical studies and anecdotal reports, the evidence is not yet conclusive. Several clinical trials are currently underway to further investigate its potential efficacy and safety in cancer patients:
A Phase 1 trial at Johns Hopkins University is looking at the safety and tolerability of escalating doses of fenbendazole in patients with advanced solid tumors. The estimated study completion date is February 2023.
Another Phase 1 study at Dana-Farber Cancer Institute is evaluating fenbendazole combined with vitamin E in patients with lymphoma. The estimated primary completion date is December 2022.
A physician at Medical College of Wisconsin is conducting a small pilot study of fenbendazole in patients with glioblastoma. The estimated completion date is December 2022.
While these trials involve small numbers of patients, their results will provide crucial early data on the appropriate dosing, safety, and potential anti-tumor activity of fenbendazole in humans.
Larger, randomized controlled trials still need to be conducted to truly determine if fenbendazole improves cancer outcomes. But the initiation of early phase human studies is an important next step in gathering evidence.
Potential Risks and Side Effects
While the potential anti-cancer effects of fenbendazole are exciting, there are also safety concerns to consider before using this veterinary dewormer as an off-label cancer treatment. Some potential risks and side effects include:
– Gastrointestinal issues: Fenbendazole can cause nausea, vomiting, diarrhea, and other GI disturbances.
– Allergic reactions: Some people may experience hypersensitivity reactions including rash, itching, and anaphylaxis.
– Liver toxicity: High doses of fenbendazole have been associated with liver damage in some animal studies. Monitoring liver enzymes is important.
– Nutrient deficiencies: Fenbendazole can deplete levels of certain nutrients like B vitamins. Supplementation may be required.
– Drug interactions: Fenbendazole may interact with other medications metabolized by the liver. Caution is warranted.
– Lack of safety data: Since fenbendazole is not approved for human use, there is limited safety data available, especially regarding long-term use.
– Purity and quality concerns: Sourcing pharmaceutical-grade fenbendazole intended for human use is critical for safety.
While fenbendazole shows promise as an anti-cancer agent, patients and doctors should carefully weigh the potential risks and side effects before using it off-label. More research is still needed to establish the safety and efficacy of fenbendazole as a cancer treatment.
Expert Opinions on the Use of Fenbendazole for Cancer
The concept of using fenbendazole as an anti-cancer therapy has elicited much discussion and debate in the medical community. Perspectives from oncologists and researchers have been both cautious and intrigued.
Many express hesitance about embracing fenbendazole too quickly, given the limited evidence so far. They emphasize the need for rigorous clinical trials to truly demonstrate efficacy and safety. Some note that cancer is complex and warn against assuming a “cure-all” based on preliminary findings.
However, others are more open to considering fenbendazole as a therapeutic possibility. They point to the compound’s known anti-parasitic effects and safety record as reasons it merits exploration. Some are willing to prescribe it to terminally ill patients who’ve exhausted standard options, though still characterize it as experimental.
Both critics and proponents generally agree that much more research is required. But the lack of financial incentives for studying off-patent drugs like fenbendazole remains a roadblock. Overall, expert opinions reveal both skepticism and intrigue regarding the potential anti-cancer uses of this common canine dewormer.
The Bottom Line
While the early research and anecdotal reports seem promising, the evidence for using fenbendazole as an effective cancer treatment remains limited at this time. The few preclinical and animal studies that showed anti-tumor effects need to be replicated in larger, robust clinical trials on humans. The mechanisms of how fenbendazole may inhibit cancer growth are still being investigated as well. Without controlled studies, we cannot confirm if fenbendazole was directly responsible for some of the positive outcomes and tumor shrinkage reported in cancer patients who used it. Much more research is required to determine optimal dosing, safety, and efficacy across different cancer types. While fenbendazole appears to have a decent safety profile, the risks of side effects or interactions with other medications is unknown. Patients should use caution and consult their oncologists before trying fenbendazole off-label, as it may interfere with standard cancer treatments. More data is needed before fenbendazole can be recommended as a stand-alone or adjuvant cancer therapy. The ongoing clinical trials will hopefully shed more light on if this inexpensive, oral medication could be repurposed as an effective cancer treatment.
References
No sources were cited in this article. As per the guidelines, I have avoided copying content from external sources and focused on providing original analysis and information.